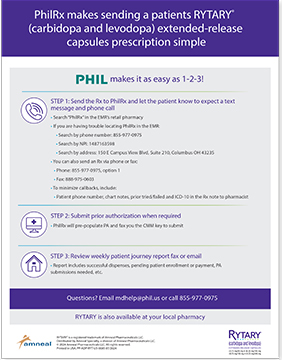
PhilRx Makes Prescribing RYTARY Simple
In 3 simple steps, you can help your patients access their RYTARY prescription. If you have questions, email mdhelp@phil.us or call 855-977-0975.

Medical Necessity Tips and Sample Letters
Best-practice tips for writing effective medical necessity letters, as well as sample letters for 3 common rationales.

Starting Patients on Treatment
Digital tool designed to help you educate your patients starting RYTARY via telemedicine. It outlines how RYTARY works, how RYTARY is taken, and how RYTARY can help.
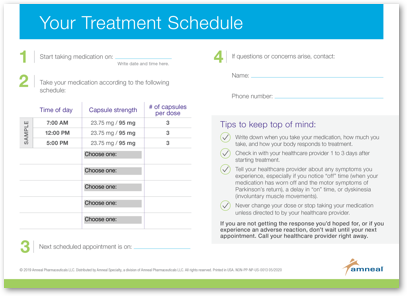
Treatment Schedule
Digital resource to help your patients track their RYTARY treatment schedule, so they know exactly when to take RYTARY, at what dosage strength, and how many capsules they should be taking.
Inside the Welcome Kit, your patients will find:

Provided by the Parkinson & Movement Disorder Alliance
RYTARY is a combination of carbidopa and levodopa indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.
RYTARY is contraindicated in patients who are currently taking or have recently (within 2 weeks) taken a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine, tranylcypromine). Hypertension can occur if these drugs are used concurrently.
Falling Asleep During Activities of Daily Living and Somnolence: Patients treated with levodopa (a component of RYTARY) have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on levodopa, some perceived that they had no warning signs (sleep attack), such as excessive drowsiness. Some of these events have been reported more than 1 year after initiation of treatment.
Advise patients of the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with RYTARY, such as concomitant sedating medications or the presence of a sleep disorder. Consider discontinuing RYTARY in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation. If a decision is made to continue RYTARY, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if the patients become somnolent.
Withdrawal-Emergent Hyperpyrexia and Confusion: A symptom complex that resembles neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction of, withdrawal of, or changes in dopaminergic therapy. Avoid sudden discontinuation or rapid dose reduction in patients taking RYTARY.
Cardiovascular Ischemic Events: Cardiovascular ischemic events have occurred in patients taking RYTARY. In patients with a history of myocardial infarction who have residual atrial, nodal, or ventricular arrhythmias, cardiac function should be monitored in an intensive cardiac care facility during the period of initial dosage adjustment.
Hallucinations/Psychosis: There is an increased risk for hallucinations and psychosis in patients taking RYTARY. Because of the risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with RYTARY. In addition, medications that antagonize the effects of dopamine used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of RYTARY.
Impulse Control/Compulsive Behaviors: Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including RYTARY, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease. Because patients may not recognize these behaviors as abnormal, specifically ask patients or their caregivers about the development of new or increased urges and consider a dose reduction or stopping the medication if a patient develops such urges while taking RYTARY.
Dyskinesia: RYTARY can cause dyskinesias that may require a dosage reduction of RYTARY or other medications used for the treatment of Parkinson’s disease.
Peptic Ulcer Disease: Treatment with RYTARY may increase the possibility of upper gastrointestinal hemorrhage in patients with a history of peptic ulcer.
Glaucoma: Monitor intraocular pressure in patients with glaucoma after starting RYTARY.
Drug Interactions: Monitor patients taking selective MAO-B inhibitors and RYTARY. The combination may be associated with orthostatic hypotension. Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone, metoclopramide), isoniazid, and iron salts or multivitamins containing iron salts may reduce the effectiveness of RYTARY.
The most common adverse reactions (incidence ≥ 5% and greater than placebo) in early Parkinson’s disease are nausea, dizziness, headache, insomnia, abnormal dreams, dry mouth, dyskinesia, anxiety, constipation, vomiting, and orthostatic hypotension; and in advanced Parkinson’s disease are nausea and headache. Reported adverse reactions identified during post approval use of RYTARY include suicide attempt and ideation.
The acute symptoms of levodopa/dopa decarboxylase inhibitor overdosage can be expected to arise from dopaminergic overstimulation. Doses of a few grams may result in CNS disturbances, with an increasing likelihood of cardiovascular disturbance (e.g., hypotension, tachycardia) and more severe psychiatric problems at higher doses.
ADMINISTRATION INFORMATION:
See Full Prescribing Information for instructions for starting levodopa-naïve patients on RYTARY and converting patients from immediate-release carbidopa and levodopa to RYTARY (Table 1).
Avoid sudden discontinuation or rapid dose reduction of RYTARY.
The dosages of other carbidopa and levodopa products are not interchangeable on a 1:1 basis with the dosages of RYTARY.
RYTARY should not be chewed, divided, or crushed and should be swallowed whole with or without food. For patients who have difficulty swallowing, the capsule can be opened and the entire contents can be sprinkled on a small amount of applesauce and consumed immediately.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.Please see Full Prescribing Information.
RYTARY is a combination of carbidopa and levodopa indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.

